Description:
1. NAME OF THE MEDICINAL PRODUCT
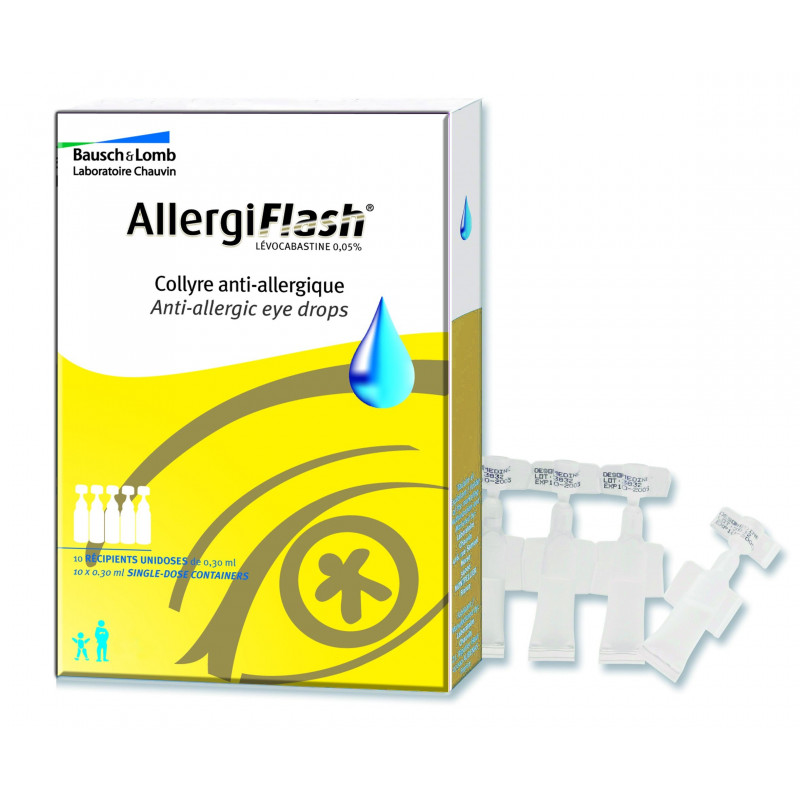
ALLERGIFLASH 0.05%, eye drop solution in single-dose container
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Levocabastine .................................................................................................................................... 0,05 g
As levocabastine hydrochloride
Per 100 ml of solution.
3. PHARMACEUTICAL FORM
Eye drops in single-dose containers.
Clear, colourless solution.
4. CLINICAL DATA
4.1. therapeutic indications
Allergic conjunctivitis.
4.2. Dosage and method of administration
Dosage:
Adult and child:
The usual recommended dose is 1 drop in each eye twice daily. This dose may be increased, if necessary, to 1 drop 3 to 4 times a day.
Treatment should not be prolonged beyond 5 days without medical advice.
Method of administration:
As with all eye drops, perform the following in order:
- Wash your hands thoroughly.
- Avoid touching the eye or eyelids with the tip of the bottle.
- Instill a drop into the eye while looking up and pulling the eyelid down slightly.
- With the eye closed, wipe off the excess, especially on the cheek.
- To avoid systemic effects, it is recommended that the inner corner of the eye be compressed for 1 minute after each instillation.
- Discard the single-dose container immediately after use.
In case of concomitant treatment with another eye drop, the instillations should be spaced out by 15 minutes.
The amount of eye drops contained in a single dose is sufficient to treat both eyes.
4.3. Contraindications
Hypersensitivity to the active substance or to any of the excipients.
4.Special warnings and precautions for use
ALLERGIFLASH 0.05% should not be used as an injection.
4.5. Interactions with other medicinal products and other forms of interaction
Not applicable.
4.6. Pregnancy and lactation
Pregnancy
No human data are available. In view of the negligible systemic transfer and the fact that no embryotoxic or teratogenic effects have been reported in animal experiments, ALLERGIFLASH 0.05% may be used with caution during pregnancy.
Lactation
Due to lack of data, the use of levocabastine is not recommended during lactation.
4.7. Effects on ability to drive and use machines
Transient visual discomfort due to slight irritation upon instillation of ALLERGIFLASH 0.05% may be experienced by the patient. In this case, it is recommended to wait until normal vision returns before driving a vehicle or operating machinery.
4.8. Undesirable effects
Adverse reactions are classified according to their frequency of occurrence as follows: very common (≥ 1/10), common (≥ 1/100, <1/10), uncommon (≥1/1000, <1/100), rare (≥1/10000, <1/1000) and very rare (<1/10000). The frequencies of postmarketing reports are not known.
Eye disorders
Rare: Mild and transient ocular irritation occurring immediately after instillation.
4.9. Overdosage
No cases of overdose have been reported.
In case of overdose, rinse thoroughly with sterile saline solution.
5. PHARMACOLOGICAL PROPERTIES
5.1. Pharmacodynamic properties
Pharmacotherapeutic class: ANTI-ALLERGIC, ATC Code: S01GX02.
- Levocabastine is a selective, potent, H1-antihistamine with a rapid and prolonged action.
- After ocular instillation, it rapidly relieves the symptoms of allergic conjunctivitis (pruritus, erythema, lacrimation, palpebral edema, chemosis) for several hours.
5.2. Pharmacokinetic properties
After ocular instillation, levocabastine is progressively and partially resorbed by the mucosa.
Plasma concentrations are too low to produce systemic effects.
5.3. Preclinical Safety Data
Non-clinical data on levocabastine from conventional oral, intravenous, dermal and ocular repeated dose toxicology studies in different animal species (rat, dog, rabbit) did not reveal any particular risk for humans.
Oral reproduction studies in mice and rats showed that levocabastine was devoid of adverse reproductive effects up to 20 mg/kg/d in rats and up to 40 mg/kg/d in mice.
Levocabastine did not show mutagenic or carcinogenic potential.
No ophthalmological abnormalities were observed in rabbits after instillation of one drop of ALLERGIFLASH 0.05% eye drops solution 4 times daily for 4 weeks.
6. PHARMACEUTICAL DATA
6.1. List of excipients
Hydroxypropyl betadex, monosodium phosphate dihydrate, disodium phosphate dodecahydrate, sodium chloride, purified water.
6.Incompatibilities
Not applicable.
6.3. Shelf life
2 years.
Use the single-dose container immediately after opening and discard after use.
6.4. Special precautions for storage
No special precautions for storage.
6.5. Nature and contents of outer packaging
Box of 10 single-dose 0.3 ml low density polyethylene containers, over-wrapped.
6.6. Special precautions for disposal and handling
No special requirements.
7. MARKETING AUTHORIZATION HOLDER
LABORATOIRE CHAUVIN
416 RUE SAMUEL MORSE
CS99535
34961 MONTPELLIER CEDEX 2
FRANCE
8. MARKETING AUTHORISATION NUMBER(S)
- 346 916-3 or 34009 346 916 3 8: 0.3 ml solution in single-dose container (PE). Box of 10.
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
[To be completed by the holder]
10. DATE OF UPDATE OF THE TEXT
[to be completed by the holder]
11. DOSIMETRY
Not applicable.
12. INSTRUCTIONS FOR THE PREPARATION OF RADIOPHARMACEUTICALS
Not applicable.
 Français
Français English
English