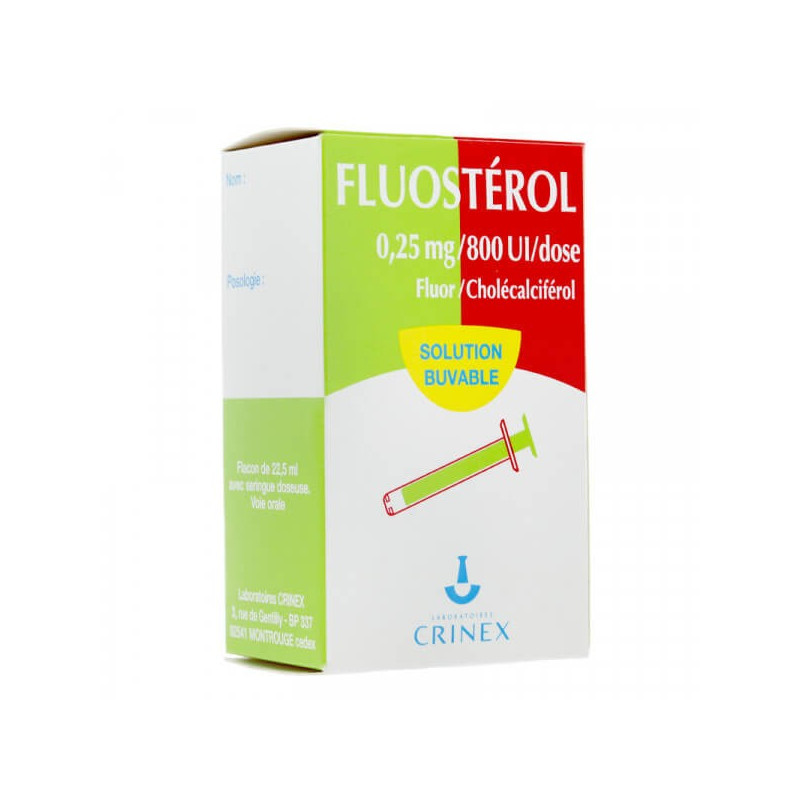
Description:
The administration of this combination is indicated for infants from birth to 18 months, as part of the joint prophylaxis of dental caries, especially for children at risk, and those whose total intake of fluoride does not reach 0.3 mg per day, but also in the case of vitamin D deficiency, in case of the use of a milk supplemented with vitamin D
The active ingredients are fluorine 0.1000 g per 100 ml of solution in the form of sodium fluoride 0.2212 g and cholecalciferol (vitamin D3) 320,000 IU in the form of cholecalciferol concentrate, powdered form 0.0080
Other components of the formulation are Alpha-tocopherol (COVIOX T 70), sodium propyl parahydroxybenzoate, sodium methyl parahydroxybenzoate, red fruit flavouring, sodium saccharin, polyoxyethylated hydrogenated castor oil (CREMOPHOR RH 40), liquid maltitol, purified water, disodium phosphate dodecahydrate, and citric acid anhydrous.
Notable excipients are polyoxyethylene castor oil, liquid maltitol, sodium methyl parahydroxybenzoate, and sodium propyl parahydroxybenzoate.
Dosage:
For infants from birth to 18 months of age, 1 dose of 0.25 ml per day should be given, which provides 0.553 mg of sodium fluoride (0.25 mg of fluoride), and 800 IU of vitamin D.
Contraindications:
Administration of this drug should be spaced from the intake of milk or milk products.
It should not be used in case of allergy to vitamin D or to one of its components, increased calcium levels in the blood or urine, kidney stones, intestinal obstruction, but also in regions where the fluoride content in the water supply is higher than 0.3 ml/L. It is recommended to ask the town hall or the DDASS in case of doubt, in order to know the local rate of fluorine in the water supply
The use of this solution is also not recommended in cases of fructose intolerance.
Fluoride supplementation in the form of medication is automatic in infants, when other sources of intake (through drinking water) are absent or insufficient. It is necessary that it is adapted to the age of the child, but also to the other potential sources such as drinking water or mineral water, fluoridated toothpastes... in order to avoid an overdose
Moreover, in case of medicated fluoride supplementation, mineralized water containing a fluoride level higher than 0.3 mg/L should not be used to prepare baby bottles
It is also important to take into account the total doses of vitamin D, especially in combination with a treatment formulated with this vitamin.
Fluosterol 0.25 mg/800 I.U./dose should not be used in combination with antacids containing magnesium or aluminium salts, nor should certain minerals such as iron, calcium or magnesium be used in combination with Fluosterol 0.25 mg/800 I.U./dose, as they may limit absorption. If you have recently or are currently taking another medication, tell your doctor or pharmacist
Pregnant and breast-feeding women are also advised to consult their doctor before taking any medication. Contents: 22.5 ml bottle with dosing syringe.
 Français
Français English
English