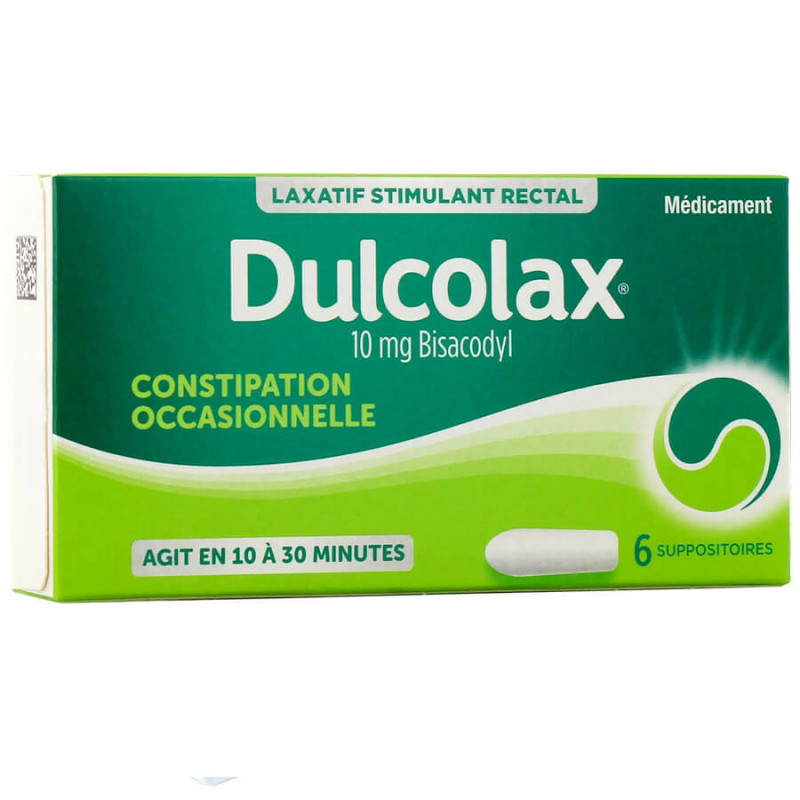
Bisacodyl............................................................................................................................. 0,01 g
For a 1.70 g suppository
4.1. Therapeutic indications
Symptomatic treatment of constipation due to rectal dyschezia.
Preparation for endoscopic examinations of the rectum.
4.2. Dosage and method of administration
Dosage
For adults only: 1 suppository per day, half an hour before the chosen time for bowel movements.
The maximum daily dose should not be exceeded.
Suppositories usually take effect between 10 and 30 minutes.
Method of administration
Suppositories should be taken out of the package and placed in the rectum, flat end first (to avoid rejection) with the tapered end down.
Because of the risk of rectal irritation, administration of the suppository should be as short as possible.
4.3. Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
Severe dehydration with electrolyte depletion.
Inflammatory diseases of the colon, ulcerative colitis, Crohn's disease.
Abdominal pain syndromes of undetermined cause, including severe abdominal pain associated with nausea and vomiting which may indicate a more serious condition.
Occlusive or subocclusive syndromes,
Children.
4.4. Special warnings and precautions for use
Warnings
The medicinal treatment of constipation is only an adjuvant to the hygienic-dietary treatment:
Enrichingthe diet with vegetable fibres and drinks
adviceon physical activity and exoneration rehabilitation.
Prolonged use (more than 10 days) is not recommended; prolonged intake can lead to:
-thelaxative disease with severe functional colopathy, rectocolic melanosis, hydroelectric anomalies with hypokalemia,
asituation of dependence with a regular need for laxatives, the need to increase the dosage and severe constipation in the event of withdrawal; this dependence, which varies according to the patient, may be created without the doctor's knowledge.
Dizziness and syncope, which may occur at the time of defecation, have been reported.
Concomitant use of other laxatives may increase the gastrointestinal side effects of Dulcolax.
Precautions for use
It is preferable not to use this drug in cases of hemorrhoidal flare-ups, anal fissures, ulcerative colitis.
4.5. Interactions with other medicinal products and other forms of interaction
Not recommended for use with
Drugs causing torsades de pointes
Antiarrhythmics: amiodarone, bretylium, disopyramide, quinidinics, sotalol.
Non-antiarrhythmics: astemizole, bepridil, erythromycin IV, halofantrine, pentamidine, sultopride, terfenadine, vincamine.
Torsades de pointes (hypokalemia is a contributing factor as well as bradycardia and a pre-existing long QT interval).
Use a non-stimulant laxative.
Combinations which are subject to precautions for use
Digitalis :
Hypokalemia promotes toxic effects of digitalis.
Monitor kalemia and, if necessary, ECG.
Use a non-stimulant laxative.
Other Hypokalemics :
Hypokalemic diuretics (alone or combined), amphotericin B (IV route), corticosteroids (gluco-mineral: systemic route), tetracosactide.
Increased risk of hypokalemia (additive effect).
Monitoring of kalemia and, if necessary, correction.
Use a non-stimulant laxative.
4.6. Fertility, pregnancy and lactation
Pregnancy
The use of bisacodyl is not recommended during pregnancy. Clinical and animal data are insufficient.
Lactation
Clinical data show that neither the active moiety of bisacodyl, BHPM (bis-(p-hydroxyphenyl)-pyridyl-2-methane), nor its glucuronides are excreted in milk in healthy nursing mothers. (Limit of detection = 1 ng/ml).
DULCOLAX may therefore be used during lactation.
Fertility
Effects on fertility have not been studied.
4.7. Effects on ability to drive and use machines
The effects of DULCOLAX on the ability to drive and use machines have not been studied.
However, patients should be informed that they may experience a vasovagal response (e.g. following abdominal spasms, defecation) which may lead to dizziness and/or syncope.
4.8. Undesirable effects
As with all laxative suppositories, prolonged use may result in anal burning and, exceptionally, congestive rectitis.
Adverse reactions are classified by system organ class and frequency according to the following convention:
Very common(³ 1/10)
Common(³ 1/100 to < 1/10)
Uncommon(³ 1/1000 to < 1/100)
Rare(³ 1/10,000 to < 1/1,000)
Very rare (< 1/10,000)
Undetermined (cannot be estimated on the basis of available data)
The most frequently reported treatment-emergent adverse events are abdominal pain and diarrhea.
Immune system disorders
Rare: Anaphylactic reactions, angioedema.
Skin and subcutaneous tissue disorders
Rare: Generalized pruritus.
Metabolism and nutrition disorders
Rare: Dehydration
Undetermined frequency: hypokalemia
Nervous system disorders
Uncommon: dizziness
Rare: Syncope
Cases of dizziness or syncope following Bisacodyl appear to be a vasovagal response (e.g., to abdominal spasms, defecation).
Gastrointestinal
Common: Abdominal pain, diarrhea, nausea.
Uncommon: Rectorrhagia, anorectal discomfort (burning and pain), vomiting.
Rare: Colitis including ischemic colitis.
Reporting of Suspected Adverse Reactions
Reporting of suspected adverse reactions after drug approval is important. It allows continuous monitoring of the benefit/risk ratio of the drug. Health professionals report any suspected adverse reaction via the national reporting system: Agence nationale de sécurité du médicament et des produits de santé (ANSM) and the network of Regional Pharmacovigilance Centres - Website: www.ansm.sante.fr.
4.9. Overdose
Symptoms in case of high doses, diarrhoea, abdominal cramps and clinically significant loss of water, potassium and other electrolytes may occur.
Chronic overdosage of DULCOLAX may result in diarrhea, abdominal pain, hypokalemia which may be associated with muscle weakness.
Correction of fluid and electrolyte disorders may be necessary in cases of severe fluid loss. This is particularly important in the elderly and in young subjects. The administration of antispasmodics may be helpful.
Renal tubular injury, metabolic alkalosis, secondary hyperaldosteronism and renal calculi have also been described in association with chronic abuse of laxatives.
5. PHARMACOLOGICAL PROPERTIES
5.1. Pharmacodynamic properties
Pharmacotherapeutic class: LAXATIVE STIMULANTS, ATC code: A06AB02
(A: Digestive system and metabolism)
Bisacodyl is a locally acting laxative, belonging to the family of phenylmethane derivatives.
It increases colonic motility and intestinal secretion of water, electrolytes and protein.
The result is a stimulation of defecation, a reduction in transit time and a softening of the stools.
Suppositories take effect in 10 and 30 minutes.
Bisacodyl stimulates the lower part of the gastrointestinal tract. Bisacodyl does not affect digestion or absorption of essential calories or nutrients in the small intestine.
5.2. Pharmacokinetic properties
After oral or rectal administration only small amounts of the drug are absorbed. Bisacodyl is rapidly hydrolysed by esterases in the intestinal mucosa to give the active ingredient bis-(p-hydroxyphenyl)-pyridyl-2-methane (BHPM) which acts locally without absorption.
The small amount absorbed is almost completely conjugated to give the inactive glucuronide BHPM.
BHPM is eliminated primarily through the feces. The form found in the urine is the glucuronide form BHPM.
The amount found in the urine is greater after oral administration than after rectal administration.
Bisacodyl partly follows the enterohepatic cycle.
5.3. Preclinical Safety Data
Signs observed in acute toxicity studies were diarrhea, decreased motor activity and piloerection in rats at 2g/kg and in dogs at 15g/kg.
Repeated dose toxicity studies in rats, minipigs and Rhesus monkeys of up to 26 weeks duration showed severe dose-dependent diarrhea in all species except the minipig. No specific histopathological changes, including nephrotoxicity, were observed. In rats treated for 32 weeks, bisacodyl induced proliferative lesions in the bladder. These lesions are considered species-specific.
Bisacodyl showed no evidence of genotoxicity in standard in-vitro genotoxicity studies.
In a carcinogenicity study conducted in p53 transgenic mice for 26 weeks, no tumors attributable to bisacodyl were observed at doses up to 8000 mg/kg/day.
Conventional carcinogenicity studies in rats were not conducted.
Teratogenicity studies in rats and rabbits were negative at doses up to 1000 mg/kg/day in rabbits (800 times the recommended dose) and in rats at up to 80 times the recommended dose.
 Français
Français English
English