Betadine Vaginal Solution 10% 125 ml
Indications :
The gynecological solution Betadine 10% is an iodinated antiseptic. This medicine is indicated in the local treatment of certain vaginal infections. It comes in a 125ml bottle.
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs

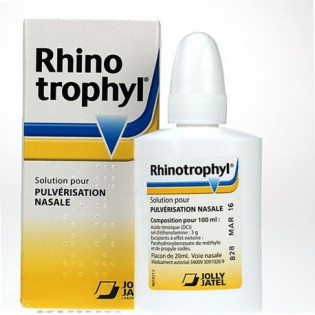
Local adjunctive treatment of nasopharyngeal mucosal disorders.
Nasal spray solution: 20 ml spray bottle with nasal tip and dip tube.
per 100 ml: Tenoic acid (INN) ethanolamine salt 3 g
Excipients: sodium hydrogen phosphate dodecahydrate, sodium hydrogen phosphate dihydrate, sodium methyl parahydroxybenzoate, sodium propyl parahydroxybenzoate, purified water.
Local adjunctive treatment of nasopharyngeal mucosal disorders.
Dosage :
Adult: 1 spray in each nostril 4 to 6 times a day.
Children over 30 months: 1 spray in each nostril 3 to 4 times a day.
Infant: 1 spray in each nostril 2 times a day.
Method of administration: Nasal use.
Nasal sprays are done with the head slightly tilted back and the bottle in an upright position.
Hypersensitivity to any of the components, especially parabens.
The indication does not justify prolonged treatment.
In the event of associated general clinical signs, systemic treatment should be considered.
Microbial contamination may occur as soon as the package is opened, and even more so the first time a nasal preparation is used.
Pregnancy:
There are no reliable data on teratogenesis in animals.
Clinically, no particular malformative or fetotoxic effects have been observed to date. However, the follow-up of pregnancies exposed to this drug is insufficient to exclude any risk.
Therefore, as a precautionary measure, it is preferable not to use this drug during pregnancy.
Breast-feeding:
Because of the lack of data on the passage of this drug into breast milk, the use of this drug should be avoided during breastfeeding.
No effect on the ability to drive and use machines has been observed.
Possible nasal irritation.
Pharmacotherapeutic class: Nasal preparation for antiseptic purposes (R: respiratory system).
Ethanolamine tenoate: for antiseptic and decongestant purposes.
Shelf life: 3 years.
Specific References
The gynecological solution Betadine 10% is an iodinated antiseptic. This medicine is indicated in the local treatment of certain vaginal infections. It comes in a 125ml bottle.
Indications: This topical medicine contains protective and healing substances. It is used in the symptomatic treatment of pain, burning and itching of the anus, especially due to hemorrhoids.
Indications : The Treasures of the Beehive
ACTIRUB® solutions (syrup and mouth spray) bring together active agents that draw all the richness and benefits of gemmotherapy*. The association of each of the constituents of the ACTI'RUB® range allows a targeted use.
*By gemmotherapy, the ACTI'RUB® solutions (syrup, mouth spray and lozenges) draw all the richness of the buds
Indications: This medication is indicated for the treatment of tobacco dependence to relieve the symptoms of nicotine withdrawal in subjects wishing to quit smoking. This medication may be used in: cases where a smoker is temporarily abstaining from smoking, a smoking reduction strategy as a step towards permanent cessation.
Promotes digestion and detoxification
Improves the functioning of the liver.
Indications: Traditionally used to relieve sore throats and/or temporary hoarseness.
- Short-term treatment of acute transient diarrhea in adults (15 years and older)
Red Tiger Balm is indicated for inflammations, muscular pains, osteoarticular, lumbar weakness and back pain.
Ialuset Hyaluronic Acid Cream 100g is a healing, repairing agent for non-infected, oozing or super-infected wounds, including leg ulcers.
The hyaluronic acid present in its formula is the main component of the fundamental substance of the dermis. Its application therefore promotes healing as soon as it is applied.
The food complement Upsa Phytovex Nez Gorge 3 actions is your ally thanks to its triple action formula:
1/ Pelargonium participates in soothing the respiratory tract and throat in case of tingling.
2/ Astragale supports the natural defenses. Vitamin D3, Selenium and Zinc contribute to the maintenance of the good functioning of the immune system. Selenium and Zinc also help protect cells against oxidative stress.
3/ Vitamin B3 (niacin) contributes to reduce fatigue and to a normal energy metabolism.
Gluten-free, lactose-free
Description: This medicine is indicated:
in the symptomatic treatment of acute diarrhea in children and infants as a complement to oral rehydration, and in adults,
Description: This medication is indicated for the treatment of sensitive vaginal yeast infections (diseases caused by microscopic fungi).
Dermal Betadine is an iodine-based antiseptic that is used for the antisepsis of superficial and small wounds or burns.
GENERIC TANAKAN
Indicated in the symptomatic treatment of certain cognitive disorders of the elderly (in particular memory disorders) with the exception of any type of confirmed dementia, disorders secondary to medication, depression or metabolic disorders.
Indications : Traditionally used to facilitate the functions of elimination of the organization.
Indications: This medicine is indicated for sore throats that are not very intense and without fever.
Nicotine EG 1mg/dose Spray solution for mouth spray is a medication used in smoking addiction, used to help smokers to quit smoking.
Oligostim Zinc - Copper is a drug recommended to modify the ground in case of functional disorders of puberty in children over 12 years, menopause or premenstrual syndrome phase.
Its formula is based on the trace elements of zinc (zinc gluconate) and copper (copper gluconate) to the tune of 0.220 mg each.