Cerulyse Ear Drops 10ml
Indications: This medication is an oily ear solution used to dissolve earwax plugs.
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs


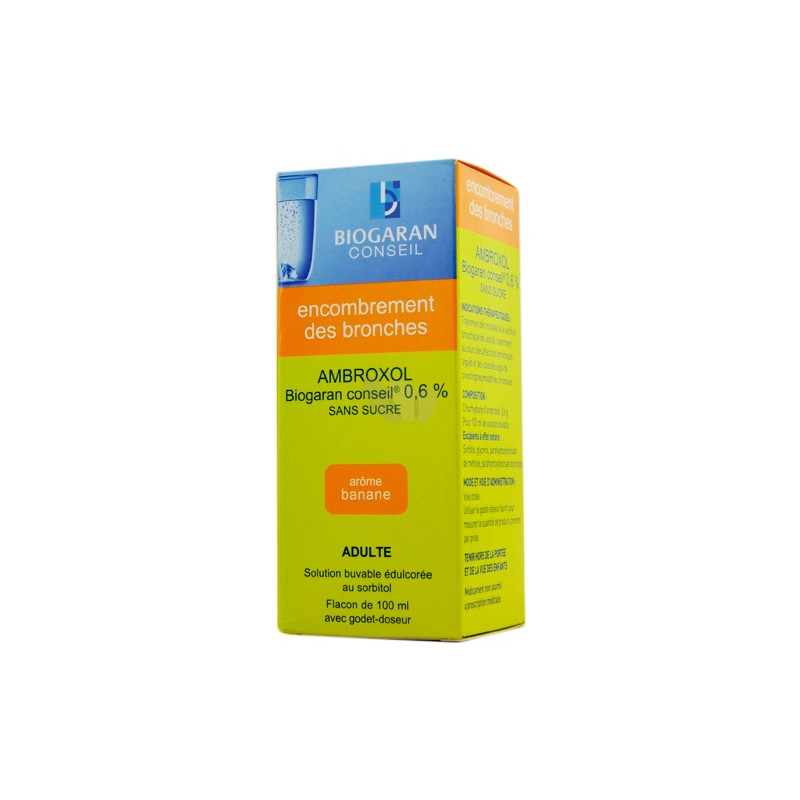
Indications: Fatty cough. Treatment of bronchial secretion disorders in adults, particularly during acute bronchial diseases.
1. NAME OF THE MEDICINAL PRODUCT
AMBROXOL BIOGARAN CONSEIL 0.6% SUGAR-FREE, drinkable solution sweetened with sorbitol
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Ambroxol hydrochloride ....................................................................................................................... 0,6 g
For 100 ml of drinkable solution.
Excipients: sorbitol, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216).
For a complete list of excipients, see section 6.1.
Oral solution.
Treatment of bronchial secretion disorders in adults, particularly during acute bronchial diseases and acute episodes of chronic lung disease.
4.2. Dosage and method of administration
RESERVED FOR ADULTS.
5 ml of oral solution contains 30 mg of ambroxol hydrochloride.
The average dosage of ambroxol hydrochloride is 60 to 120 mg per day divided into 2 doses, i.e. 5 to 10 ml twice a day (morning and evening).
A measuring cup graduated at 2.5 ml, 5 ml, 7.5 ml, 10 ml and 15 ml or a 5 ml measuring spoon are provided to measure the quantity of active ingredient for one dose:
Either 5 ml to 10 ml from the measuring cup or 1 to 2 measuring spoons.
History of hypersensitivity reactions to any of the constituents.
4.4. Special warnings and precautions for use
The combination of a bronchial mucosifier with a cough suppressant and/or secretion drying substances (atropine) is not recommended.
This medicine contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) and may cause allergic reactions (possibly delayed).
This medicine contains sorbitol. Its use is not recommended in patients with fructose intolerance.
Due to the presence of sorbitol, this medicine may cause a moderate laxative effect.
4.5. Interactions with other medicinal products and other forms of interaction
Not applicable.
Pregnancy
Animal studies have not shown any evidence of a teratogenic effect. In the absence of a teratogenic effect in animals, a malformative effect in humans is not expected.
Indeed, to date, substances responsible for malformations in humans have been shown to be teratogenic in animals in well-conducted studies in two species.
Clinically, there are currently no sufficiently relevant data to assess a possible malformative or fetotoxic effect of ambroxol hydrochloride when administered during pregnancy.
Therefore, as a precautionary measure, ambroxol hydrochloride should not be used during pregnancy.
Breastfeeding
The use of this product is not recommended when breastfeeding.
4.7. Effects on ability to drive and use machines
Not applicable.
Minor gastrointestinal effects such as nausea, vomiting, gastralgia, pyrosis, dyspepsia have been reported infrequently.
- Have been described:
o cases of mucocutaneous reactions such as erythema, rash, pruritus, urticaria;
o very rarely, anaphylactoid reactions with shock and angioedema, which have had a favorable outcome in reported cases.
In these cases, treatment must be discontinued.
- Headache and dizziness have also been reported very rarely.
In case of overdose, treatment should be symptomatic.
5.1. Pharmacodynamic properties
Pharmacotherapeutic class: MUCOLYTICS, ATC Code: R05CB06.
Ambroxol has mucokinetic and expectorant properties.
It stimulates, by its action on the secretory cells, the bronchial secretion and promotes the production of a more mobilizable mucus. It increases ciliary activity.
5.2. Pharmacokinetic properties
Ambroxol is well absorbed orally. Peak plasma levels are reached in approximately two hours.
Bioavailability is approximately 70%.
High volumes of distribution indicate significant extravascular distribution.
The elimination half-life averages 7.5 hours. Elimination is predominantly urinary, with two major metabolites excreted as glucuronide conjugate.
Not applicable.
Glycerol, citric acid monohydrate, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), banana flavour, liquid sorbitol (non-crystallisable), purified water.
Not applicable.
2 years.
6.4. Special precautions for storage
No special precautions for storage.
6.5. Nature and contents of outer packaging
100 ml or 150 ml in a bottle (type III brown glass) closed with a stopper (Polyethylene) fitted with a seal (Polyethylene) with a measuring cup (Polypropylene) graduated at 2.5 ml, 5 ml, 7.5 ml, 10 ml and 15 ml or a measuring spoon (Polystyrene) graduated at 2.5 ml and 5 ml.
6.6. Special precautions for disposal and handling
No special requirements.
7. MARKETING AUTHORIZATION HOLDER
BIOGARAN
15 BOULEVARD CHARLES DE GAULLE
92700 COLOMBES
8. MARKETING AUTHORISATION NUMBER(S)
- 361 787-6: 150 ml bottle (glass) + measuring cup (Polypropylene).
- 361 920-8: 150 ml bottle (glass) + measuring spoon (Polystyrene).
- 381 268-4 or 34009 381 268 4 6: 100 ml bottle (glass) + measuring spoon (Polystyrene).
- 381 415-7 or 34009 381 415 7 3: 100 ml bottle (glass) + dosing cup (polypropylene).
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
[To be completed by the holder]
10. DATE OF UPDATE OF THE TEXT
[To be completed by the holder]
Not applicable.
12. INSTRUCTIONS FOR THE PREPARATION OF RADIOPHARMACEUTICALS
Indications: This medication is an oily ear solution used to dissolve earwax plugs.
GeloVox 20 Pastilles is a food supplement in the form of pastille, intended to relieve hoarseness and extinctions of voice.
Refresh Collyrium is an ophthalmic medication indicated in dry eye to relieve signs of irritation of the eye, in case of insufficient tears.
The other components are sodium chloride, sodium hydroxide or hydrochloric acid (to adjust the PH), purified water.
Zinc granions are used in adults to rebalance the oligo-elements in the human body, they are used in particular in inflammatory acne, acrodermatitis and enteropathy
Description: This medicine is used as an adjunct to the treatment of benign acute bronchial diseases in adults aged 15 years and over.
The duration of treatment is limited to 3 days.
Prospan sugar-free syrup is a herbal medicine based on climbing ivy, traditionally used in case of productive cough inadults,teenagers andchildren over 2 years old.
Packaging : 100 ml bottle with measuring cup
Description: Soothing - regenerating essential oil.
Indications: Squash is known for its effect on male urinary comfort, helping to maintain normal urinary flow.
Indications
This medicine is recommended in chronic inflammatory conditions of the upper respiratory tract: chronic rhinitis and rhinopharyngitis.
From 5 years old
ImmuVit'4G Senior
Multivitamins and immunity
12 vitamins, 7 minerals, 4 ferments, Immu4G complex
30 triple release tablets
Food supplement
Indications: This medicine contains a non-steroidal anti-inflammatory drug: ibuprofen. It is indicated for adults and children weighing more than 30 kg (approximately 11-12 years), for the short-term treatment of fever and/or pain such as headaches, flu, toothache, aches and pains, painful periods.
Indications: This medicine is an antispasmodic. It fights against abnormal and painful contractions of the intestine, the bile ducts, the urinary tract and the uterus.
Cleanses and clears the nose in case of colds and allergic rhinitis. Adults, children and babies from 3 months. Preservative-free, 100% natural, made from seawater and purified water. Made in France.
Description: Essential oil dedicated to the respiratory sphere, antiviral, anti-inflammatory, mucolytic, expectorant
Oropolis throat spray is a food supplement based on propolis
Sugar free
Packaging: 20 ml bottle
2 to 3 sprays up to 4 times a day.
Bulking agent: glycerol; hydroalcoholic extract of propolis (38%) containing 12% propolis.
Description:
Used as part of an immediate stop smoking, NICORETTESKIN® patches allow you to stop smoking without thinking about it. First step for smokers with a moderate to low dependency.