MUCODRILL ACETYLCYSTEINE 600MG 10 EFFERVESCENT CPS ORANGE FLAVOUR
Description : The acetylcysteine contained in this medicine is a mucolytic. It thins the mucous secretions of the bronchial tubes to make them easier to evacuate by coughing.
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs

Syrup indicated for the treatment of bronchial secretion disorders, particularly during acute bronchial disorders: acute bronchitis and acute episodes of chronic bronchopneumopathy.
Indication: Oily cough
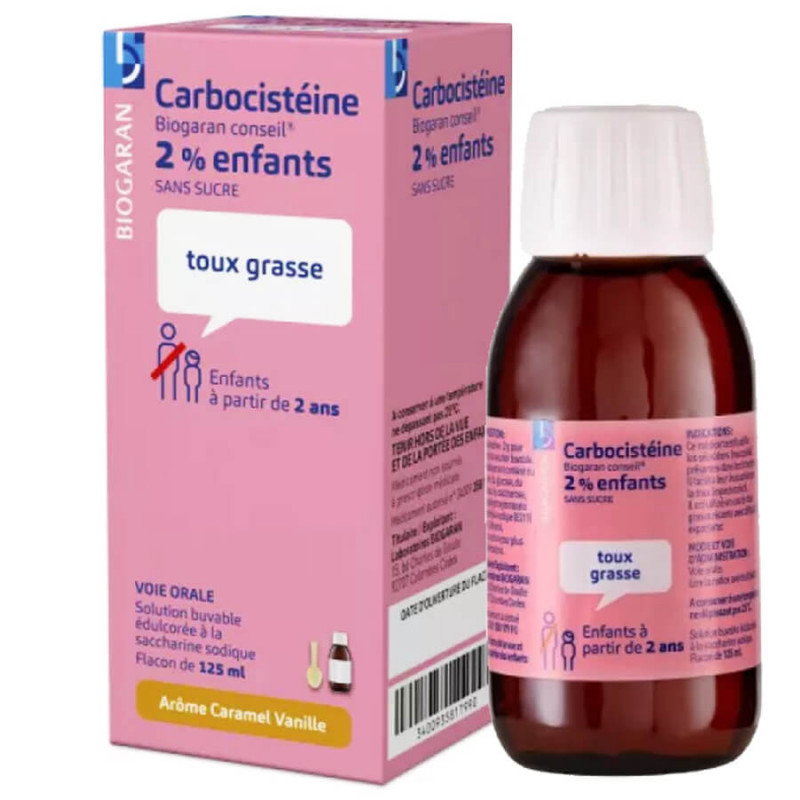
Carbocisteine Biogaran 2% coux grasse enfants Sans Sucre 125 ml is indicated for the treatment of bronchial secretion disorders, particularly during acute bronchial conditions: acute bronchitis and acute episodes of chronic bronchopneumopathy.
Dosage
This speciality is suitable for patients on a hypoglucidic or hypocaloric diet.
One 5 ml measuring spoon contains 100 mg carbocisteine.
Pediatric population
Children aged 2 to 5
200 mg daily, divided into 2 doses, i.e. 1 measuring spoon of 5 ml twice a day.
Children over 5 years of age
300 mg daily, divided into 3 doses, i.e. 1 measuring spoon of 5 ml 3 times a day.
Duration of treatment
8 to 10 days.
Method of administration: Oral.
oral solution: Carbocisteine 2 g/100 ml.
Excipients : Hydroxyethylcellulose, Sodium saccharin, Sodium methyl parahydroxybenzoate (E 219), Caramel flavoring (1), Vanilla flavoring (2), (Cocoa alcohol, Cocoa spirit, Grape alcohol, Grape spirit, Caramel sugar, (Saccharose, Fructose, Galactose), Vanilla extract, Vanillin, Coffee extract, Anisic aldehyde, Propylene glycol, Ethyl alcohol), Sodium hydroxide, Purified water.
1 measuring spoon of 5 ml contains 100 mg carbocisteine.
1 measuring spoon of 5 ml = 0.24 Kcal.
Notable excipients: glucose, fructose, sucrose, sodium methyl parahydroxybenzoate (E219) and ethanol.
Contraindications:
- Hypersensitivity to carbocistein, methyl parahydroxybenzoate, other parahydroxybenzoate salts or any of the excipients listed under Excipients.
- In case of active peptic ulcer.
- Infants (under 2 years of age) (see Warnings and precautions for use).
Warnings and other special precautions for use:
In the event of oily, purulent sputum, fever or chronic bronchial or lung disease, re-examine the clinical situation.
Productive coughs, which represent a fundamental element of bronchopulmonary defense, should be respected.
The combination of bronchial mucomodifiers with cough suppressants and/or secretion-drying substances (atropinics) is irrational.
Mucolytics can induce bronchial overcrowding in infants. Their capacity to drain bronchial mucus is limited by the physiological characteristics of the respiratory tree. They should therefore not be used in infants (see Contraindications and Adverse reactions).
Treatment should be reassessed in the event of persistent or worsening symptoms or pathology.
Caution is advised in elderly patients, in patients with a history of peptic ulcers, or in patients taking drugs that may cause gastrointestinal bleeding.
If such bleeding occurs, patients should discontinue treatment.
This medicine contains sucrose. Patients with fructose intolerance, glucose-galactose malabsorption syndrome or sucrase/isomaltase deficiency (rare hereditary diseases) should not take this medicine.
This medicine contains glucose. Patients with glucose-galactose malabsorption syndrome (a rare hereditary disease) should not take this medicine.
This medicine contains fructose. This medicine contains less than 20 mg fructose per 5 ml measuring spoon. The additive effect of concomitantly administered products containing fructose (or sorbitol) and dietary intake of fructose (or sorbitol) must be taken into account.
This medicine contains "parahydroxybenzoate" (E219) and may cause allergic reactions (possibly delayed).
This medicine contains less than 1 mmol (23 mg) per 5 ml measuring spoon, i.e. it is essentially "sodium-free".
This medicine contains 30 mg alcohol (ethanol) per 5 ml measuring spoon. The amount per measuring spoon is equivalent to less than 1 ml beer or 1 ml wine. The small amount of alcohol contained in this medicine is unlikely to have any noticeable effect.
Pregnancy and breast-feeding :
Pregnancy
Animal studies have shown no evidence of teratogenic effects.
No data are available on the use of carbocisteine in pregnant women.
Consequently, the use of carbocisteine in pregnant women is not recommended.
Breast-feeding
No data are available on the passage of carbocisteine into breast milk.
Consequently, the use of carbocisteine in breast-feeding women is not recommended.
Effects on ability to drive and use machines :
Not applicable.
Description : The acetylcysteine contained in this medicine is a mucolytic. It thins the mucous secretions of the bronchial tubes to make them easier to evacuate by coughing.
Hexaphyto adult cough syrup is a medical device for adults. It is used in the event of a dry or oily cough to provide rapid relief and restore respiratory comfort.
Description : STODALINE homeopathic syrup without sugar and without alcohol for dry coughs and fatty coughs.
Carbocisteine Mylan 2% Children's sugar-free syrup, cough, drinkable solution sweetened with liquid maltitol and sorbitol is a medicine to thin bronchial secretions, indicated for coughing up mucus.
This syrup is indicated in case of bronchial congestion, in particular during acute episodes of bronchitis.
Syrup to relieve dry and hacking coughs in adults and children aged 1 and over. Made with 100% natural active ingredients (honey, mallow, tamarind).
Phytovex Syrup Cough Mixed without sugar is a medical device to relieve and alleviate the cough (dry, fatty, allergic or irritative) and the sore throat.
The Hexaphyto brand offers its cough spray, a medical device for adults and children aged 8 and over. The spray provides rapid relief from coughs and irritation, without causing drowsiness.
Surbronc expectorant ambroxol 30 mg scored tablet is a medicine used in the treatment of bronchial secretion disorders in adults, especially during acute bronchial diseases and acute episodes of chronic lung disease.
This medicine is an expectorant. It facilitates the evacuation of bronchial secretions by coughing.
Indications: Treatment of bronchial secretion disorders in adults, especially during acute bronchial diseases and acute episodes of chronic bronchial diseases.
This medicine is an expectorant. It facilitates the evacuation of bronchial secretions by coughing.
Recommended for bronchial congestion, particularly during acute episodes of bronchitis.
raspberry aroma
100 ml bottle.
3C Pharma Sédatuxil Syrup 125 ml is a food supplement in the form of syrup, suitable for the whole family (children from 36 months). This syrup calms coughs.
It acts to facilitate breathing, to alleviate the throat, the pharynx and the respiratory tracts, to calm the mucous membranes.
5 extracts of plants specifically selected, of which:
- marshmallow, which relieves and soothes in case of dry cough and irritation of the throat,
- the plantain which alleviates in the event of irritation of the throat and the pharynx,
- the wallflower which contributes to maintain the respiratory tracts in good health.
Indication: Adults and children from 36 months.
Boiron Mandarin Sucking Paste is a homeopathic medicine traditionally used as an adjuvant in the treatment of coughs.
Description: Homeopathic medicine traditionally used in the symptomatic treatment of hoarse cough and hoarseness.
Indications: This medicine is a fluidifier of bronchial secretions, it facilitates their evacuation by coughing. This medicine is recommended in the states of congestion of the bronchi, particularly during acute episodes of bronchitis.
Prospan sugar-free syrup is a herbal medicine based on climbing ivy, traditionally used in case of productive cough inadults,teenagers andchildren over 2 years old.
Packaging : 100 ml bottle with measuring cup
Naturactive Sirop Aux Essences Toux Sèche & Grasse 120 ml is a medical device indicated as an adjuvant in the treatment of the dry and fatty cough, as well as in the event of irritation of the oropharyngeal mucous membrane.
Indication : Treatment of the fatty and dry cough. For adults and children from 12 months.