
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs

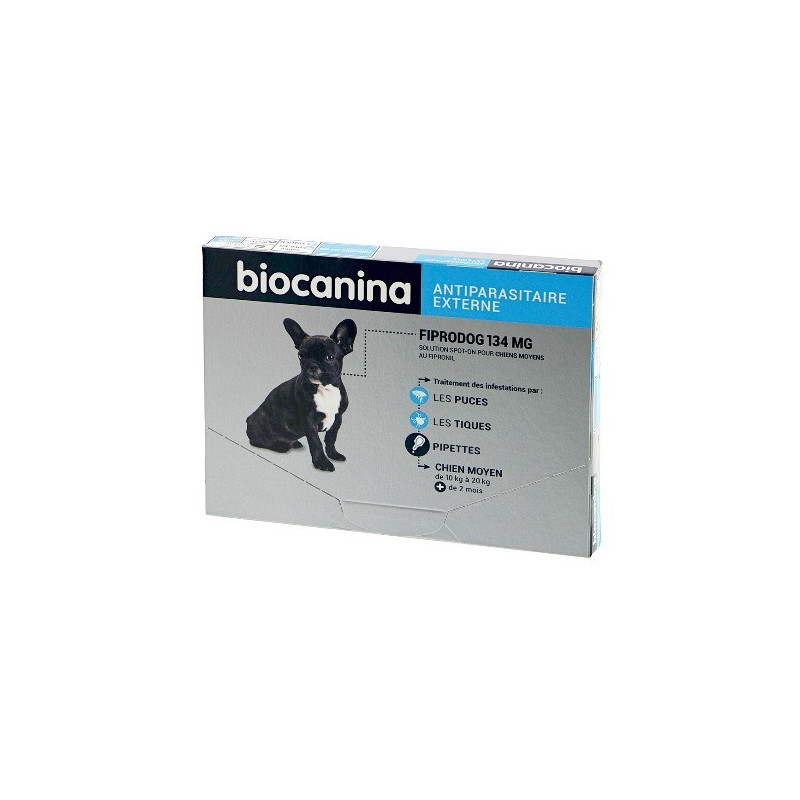
Fipronil-based flea and tick control pipettes for medium dogs weighing 10 to 20 kg
One 1.34 mL pipette contains:
Active substance(s): Fipronil ..................................................134.000 mg
Excipient(s): Butylated hydroxyanisole (E 320) 30.268 mg
Butylated hydroxytoluene (E 321) ................................... 50.134 mg
For a complete list of excipients, see section "List of excipients".
Spot-on solution.
Clear, colourless to yellowish solution.
4.1. target species
Dogs (10-20 kg).
4.2. indications for use, specifying target species
In dogs (10-20 kg):
- Treatment of flea infestations(Ctenocephalides spp.).
Insecticidal efficacy prevents further flea infestation for 6 weeks.
The drug can be incorporated into a treatment program for Flea Bite Allergy Dermatitis (FBAD), when this diagnosis has been made by a veterinarian.
Although the product does not always show immediate acaricidal efficacy (ticks may be present after 48 hours), it has persistent acaricidal activity for up to 4 weeks against Dermacentor variabilis and up to 3 weeks against Rhipicephalus sanguineus.
4.3. Contraindications
Do not use in puppies under 2 months of age and/or weighing less than 2 kg, as no data are available.
Do not use in sick (systemic disease, fever, etc.) or convalescent animals.
Do not use in rabbits as adverse, sometimes lethal, effects may occur.
This veterinary medicinal product has been developed specifically for dogs. Do not use in cats to avoid overdose.
Do not use in case of hypersensitivity to the active substance or to any of the excipients.
4.4. warnings specific to each target species
The veterinary medicinal product does not prevent tick infestations in animals.
Ticks are generally killed within 48 hours of the animal's infestation; however, it is possible that ticks (living or dead) may still be attached after this time. Some will have already had a blood meal.
Ticks usually die before they are fully engorged with blood, so the risk of transmitting infectious diseases from them is minimized, although it cannot be totally excluded. Once dead, the ticks fall off the animal immediately; those that remain attached can be removed by gently tugging on them.
To best control fleas when there are several animals in the same household, all dogs and cats should be treated with an authorised insecticide.
Animal fleas often infest the animal's basket, sleeping area and resting areas such as carpets, sofas, etc. In case of massive infestation and from the beginning of pest control measures, these areas should be treated with a suitable insecticide and vacuumed regularly.
Medicated shampooing, followed by thorough drying of the animal, 1 to 2 hours before application of the treatment and weekly bathing of the animal for 6 weeks do not affect the effectiveness of this veterinary drug against fleas. Avoid bathing or immersion in water for two days after application of the treatment.
Used as part of a therapeutic program to control allergic dermatitis to flea bites, monthly applications to allergic dogs and other household pets are recommended.
4.5. Special precautions for use
i) Special precautions for use in animals
Do not use this veterinary drug in dogs weighing less than 10 kg.
It is important to ensure that the product is applied to an area where the animal can not lick. Ensure that newly treated animals do not lick each other.
Avoid contact with the animal's eyes. In case of accidental contact of the veterinary medicine with the eyes, wash immediately with plenty of water.
Do not apply the veterinary medicine on wounds or skin lesions.
It is possible that some ticks remain attached. The risk of transmission of infectious diseases cannot be completely excluded under unfavourable conditions.
(ii) Special precautions to be taken by the person administering the veterinary medicinal product to animals
This product may cause irritation to mucous membranes and eyes. Therefore, contact of the product with the mouth and eyes should be avoided.
In case of accidental contact with the eyes, rinse immediately with plenty of water. If eye irritation persists, seek medical advice immediately and show the instructions or labelling.
Avoid contact with fingers. If contact occurs, wash fingers immediately with soap and water.
Wash hands after use.
Do not smoke, drink or eat while applying the product.
Persons with a known hypersensitivity to fipronil or any of the excipients should avoid contact with this veterinary drug.
Do not handle treated animals and do not allow children to play with treated animals until the application site has dried. It is therefore recommended that animals not be treated during the day but rather in the early evening. In addition, it is advisable not to allow treated animals to sleep with their owners, especially children.
Keep pipettes in the original packaging and dispose of used pipettes immediately.
iii) Other precautions
Do not allow dogs to swim in waterways for 2 days after application (see section "Special precautions for disposal of unused drugs or wastes derived from the use of these drugs").
4.6. Adverse reactions (frequency and severity)
In case of licking, a brief episode of hypersalivation may be observed, mainly related to the nature of the solvent.
Among the adverse reactions, very rare cases of transient skin reaction at the application site (desquamation, local alopecia, pruritus, erythema) as well as generalized pruritus or alopecia have been reported after use.
Very rarely, hypersalivation, reversible neurological symptoms (hyperesthesia, depression, nervous symptoms), vomiting or respiratory signs have been observed after use.
The frequency of adverse reactions is defined using the following convention:
Very common (adverse reactions in more than 1 in 10 animals during treatment)
Common (between 1 and 10 animals in 100)
Uncommon (between 1 and 10 animals in 1,000)
Rare (between 1 and 10 animals in 10,000)
Very rare (less than 1 in 10,000 animals, including isolated cases)
4.7. Use during pregnancy, lactation or oviposition
Laboratory studies with fipronil have not shown teratogenic or embryotoxic effects.
No studies with this drug have been conducted in pregnant or lactating animals. The use of the product during gestation or lactation should be made only after evaluation of the benefit/risk ratio by the attending veterinarian.
4.8. Drug and other interactions
None known.
4.9. Dosage and administration
Route of administration and dose:
For local application to the skin only. For external use only.
Animals should be accurately weighed prior to treatment.
One 1.34 mL pipette will treat a dog weighing between 10 and 20 kg; this corresponds to a recommended minimum dose of 6.7 mg fipronil per kg body weight.
The minimum interval between applications should not be less than 4 weeks.
For safe application:
Detach one of the blisters from the blister pack. This prevents accidental opening of the rest of the blister pack, which protects the unopened pipettes from moisture. Open the blister with scissors. To avoid damaging the pipette, cut along the line marked with a pair of scissors. Carefully remove the foil from the cut end and take out the pipette.
Hold the pipette upright. Tap the pipette lightly to draw all the liquid contents into the pipette body. Fold the top part of the pipette backwards. The pipette can now be tilted, if necessary. To open the pipette, break off the tip by bending it sharply at the pre-cut line.
Spread the hair between the shoulder blades so that the skin is visible. Place the tip of the pipette on the skin and squeeze the pipette several times to empty the entire contents directly onto the skin at one point.
Applying the solution close to the base of the head reduces the risk of the animal licking the solution. Ensure that animals do not lick each other after treatment.
Care should be taken to avoid over-wetting the coat with the product, as this would result in a sticky appearance of the treated area. However, if this is the case, this appearance will disappear within 24 hours of application.
4.10. Overdose (symptoms, emergency procedures, antidotes), if necessary
The toxicity of the veterinary drug applied to the skin is very low. However, the risk of adverse reactions (see adverse reactions) may increase in case of overdose, which means that animals should always be treated with the pipette size corresponding to their weight.
Specific safety studies after repeated administration or in case of overdose have not been conducted, as the toxicological profile of the active substance and the excipients is known.
4.11. withdrawal period
Not applicable.
Pharmacotherapeutic group: external antiparasitic agents, fipronil.
ATC-vet code: QP53AX15.
5.1. Pharmacodynamic properties
Fipronil is an insecticide and acaricide belonging to the phenylpyrazole family. It acts by inhibiting the GABA complex, binding to the chlorine channel and thus blocking the pre- and post-synaptic passage of chloride ions across the cell membrane. This results in uncontrolled central nervous system activity and death of insects and mites.
Fipronil has insecticidal activity against fleas(Ctenocephalides spp.) and acaricidal activity against ticks(Rhipicephalus sanguineus and Dermacentor variabilis.)
Fleas are killed within 48 hours. Most ticks are killed within 48 hours. Some ticks may still be present 48 hours after treatment.
5.2. Pharmacokinetic characteristics
The veterinary drug is distributed throughout the skin of the animal within 48 hours.
Absorption of fipronil is negligible in dogs after topical application.
The concentration of fipronil in the coat decreases with time.
6.1. List of excipients
Butylated hydroxyanisole (E320)
Butylated hydroxytoluene (E321)
Diethylene glycol monoethyl ether
6.2. major incompatibilities
None known.
6.3. Shelf life
Shelf life of the veterinary medicinal product as packaged for sale: 30 months.
6.4. Special precautions for storage
This medicinal product does not require special precautions for storage.
Keep in the original packaging.
6.5. Nature and composition of the immediate packaging
Polyethylene terephthalate/polypropylene pipette - polyethylene terephthalate/aluminium
6.6. Special precautions for disposal of unused veterinary medicinal products or waste products derived from the use of these medicinal products
Fipronil may harm aquatic organisms. Do not contaminate ponds, streams or ditches by disposing of the product or its empty packaging.
Empty packaging and any leftover product should be disposed of in accordance with current waste regulations.
In cats and dogs: Treatment of infestations by fleas (Ctenocephalides spp.), by ticks (Ixodes ricinus, Rhipicephalus sanguineus), by chewing lice in dogs (Trichodectes canis) and cats (Felicola subrostratus).
Insecticide diffuser based on active ingredients of plant origin, effective for basic household treatment in the event of pest infection. Immediate and long-lasting action inside the home, for up to 4 months. Application forbidden on and in the presence of animals.
Biocanina 's Serenity anti-stress spray is a product specially developed for the well-being of cats aged six weeks and over. It is particularly recommended for a targeted action during a stressful situation such as a trip, a visit to the vet, the arrival of a child or a new animal, changes in the environment, strange noises or smells
Prevention and treatment of flea infestations (Ctenocephalides felis).
Indications: ECO-LOGIS SPRAY has a powerful and prolonged action against insects (fleas, lice, bedbugs), mites (ticks, augusts, scabies agents) and spiders. Permethrin quickly destroys adult parasites. Methoprene inhibits the development and metamorphosis of larvae and the fertility of females.
Description: Large display thermometer with flexible probe
Biocanina ear milk, a milk for cleaning and hygiene of the ear canal of dogs and cats.
Dog and cat ear hygiene and cleaning.
Indications : Cleanse the air, clear the respiratory tract, limit sources of allergies (children's and adults' bedrooms, sick people, damp rooms, toilets, kitchen...), polluted or smoky atmosphere, odours from bedding, animals, offices, workshops, public places, cars...
In polyparasitic dogs, curative treatment of infestations by the following parasites:
- Adult and immature gastrointestinal nematodes: Trichuris vulpi, Toxocara canis, Toxascaris leonina, Ancylostoma caninum, Uncinaria stenocephala.
- Adult and immature cestodes: Taenia spp, Dipylidium caninum.
Vinyl gloves NON powdered Non Sterile Vinyl examination gloves
Non Sterile - Non Powdered
Complies with the 93/42/CE directive - SQL 1,5 standard Box of 100 gloves
Dewormer against roundworms and tapeworms.
For small dogs and puppies (over 2 weeks) from 0.5kg to 10kg.
Meat flavour
The Biocanina anti-stress diffuser is an ideal accessory for kitten owners. It allows you to soothe and reduce stress-related behaviours in cats from the age of 6 weeks thanks to active ingredients of natural origin which spread calming and reassuring properties in the air
Biocanina has been passionately caring for dogs and cats for over 50 years. Biocanina has developed several products related to animal reproduction, including an anti-lactation product for the symptomatic treatment of dry milk production in cats and bitches.
Female dog and female cat.
Non-hormonal drying-up of milk secretion.
The URGO Spray dressing is indicated in the case of superficial wounds, scratches, abrasions. It can be applied to all areas of the body, even particularly difficult ones: elbows, knees, fingers. From the age of 3, this spray bandage can be used to treat all the family's injuries.
Indications: This medicine is indicated in the treatment of dyspeptic disorders (slow digestion, bloating).
Neurogenius Senior is a food supplement based on gingko biloba, bacopa, magnesium, resveratrol and vitamins that helps to
- to support the memory and the whole of the cognitive functions,
- to maintain good mental performances,
- guaranteed stable psychological functions.
Cold, Rhinitis, Rhinophagyngitis - Adults
Urgo Urgosyval Plaster Woven White 5 m x 2.5 cm is a medical device indicated for the fixing of bandages.
Medical device on rubbing/pulling area. General public use.
Contains rosin derivatives which can cause a cutaneous allergy.
Indication : White woven plaster.